Confinement studies
Within our GRK on confinement-controlled chemistry we attempt to understand how a confinement by reverse micelles controls chemical reactions. These assemblies of nanoscale water pools solubilized by a layer of surfactants in nonpolar solvents find application in many areas of science and technology. For instance, they can be employed as chemical and enzymatic nanoreactor,[1] in nanoparticle synthesis[2] or as experimental models for physically confined systems with limited amounts of water.[3] Understanding the composition, size and morphology of micellar structures can improve the applications further. We study these systems by scattering techniques such as dynamic light scattering (DLS), small angle scattering (SAXS), UV-Vis, and FTIR spectroscopy.
Students working on this project: Lisa Schardt & Patricia Gnutt
Collaborations: Prof. Tschulik (RUB), BioSAXS team at DESY
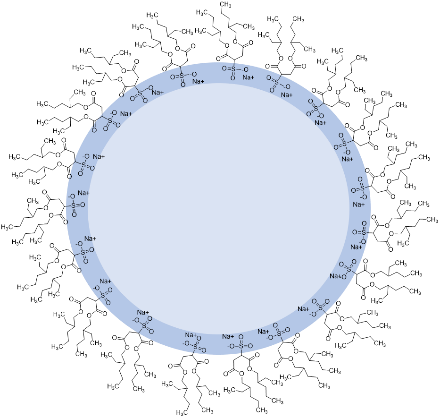
Reverse micelle of the surfactant AOT as a model system to study confinement.
[1] Petit, C., Lixon, P. & Pileni, M. In Situ Synthesis of Silver Nanocluster in AOT Reverse Micelles. 12974–12983 (1993), DOI: 10.1021/j100151a054
[2] Zan, G. & Wu, Q. Biomimetic and Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Mater. 28, 2099–2147 (2016), DOI: 10.1002/adma.201503215
[3] Senske, M., Smith, A. E. & Pielak, G. J. Protein Stability in Reverse Micelles. Angew. Chemie – Int. Ed. 55, 3586–3589 (2016), DOI: 10.1002/anie.201508981